
#Inr normal range 4 update
( TN 1388) ( TN 1388) (CR 8691)Ġ8/2015 - This change request (CR) is the 3rd maintenance update of ICD-10 conversions/updates specific to national coverage determinations (NCDs). These updates do not expand, restrict, or alter existing coverage policy.
#Inr normal range 4 code
( TN 1199) ( TN 1199) (CR 8197)Ġ5/2014 - CMS translated the information for this policy from ICD-9-CM/PCS to ICD-10-CM/PCS according to HIPAA standard medical data code set requirements and updated any necessary and related coding infrastructure. ( TN 1165) (CR8109)Ġ3/2013 - CMS translated the information for this policy from ICD-9-CM/PCS to ICD-10-CM/PCS according to HIPAA standard medical data code set requirements and updated any necessary and related coding infrastructure. ( TN 90) (CR6138)Ġ1/2013 - CMS translated the information for this policy from ICD-9-CM/PCS to ICD-10-CM/PCS according to HIPPA standard medical data code set requirements and updated any necessary and related coding infrastructure. ( TN 156) (CR 2071)Ġ7/2008 - Medicare will cover the use of home PT/INR monitoring for chronic, oral anticoagulation management for patients with mechanical heart valves, chronic atrial fibrillation, or venous thromboembolism (inclusive of deep venous thrombosis and pulmonary embolism) on warfarin. This national coverage determination (NCD) is distinct from, and makes no changes to, the PT clinical laboratory NCD at section 190.17 of Publication 100-03 of the NCD Manual.Ġ5/2002 - Provided coverage for patients with mechanical heart valves when certain conditions have been met under the Medicare program. All other indications for home PT/INR monitoring not indicated as nationally covered above remain at local Medicare contractor discretion.Ģ.

#Inr normal range 4 portable
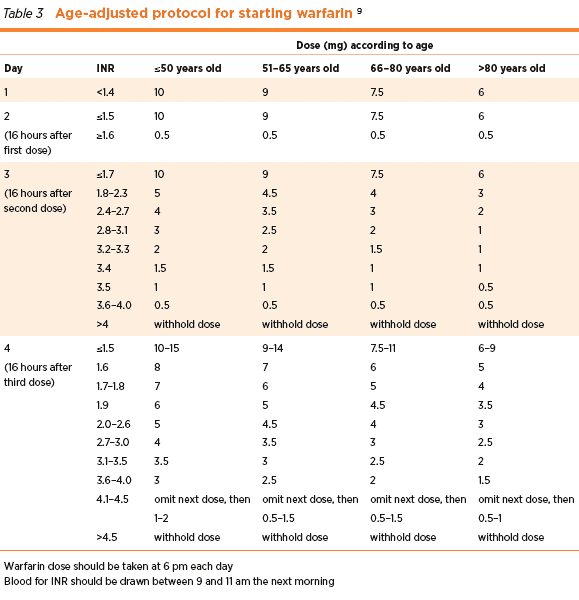
A PT/INR monitoring system is a portable testing device that includes a finger-stick and an FDA-cleared meter that measures the time it takes for a person’s blood plasma to clot.įor services furnished on or after March 19, 2008, Medicare will cover for the use of home PT/INR monitoring forchronic, oral anticoagulation management for patients with mechanical heart valves, chronic atrial fibrillation, or venous thromboembolism (inclusive of deep venous thrombosis and pulmonary embolism) on warfarin.

For this reason, since October 4, 2006, it falls under the category of a Food and Drug Administration (FDA) “black-box” drug whose dosage must be closely monitored to avoid serious complications. It is widely used for various medical conditions, and has a narrow therapeutic index, meaning it is a drug with less than a 2-fold difference between median lethal dose and median effective dose. Warfarin (also prescribed under other trade names, e.g., Coumadin®) is a self-administered, oral anticoagulant (blood thinner) medication that affects the vitamin K-dependent clotting factors II, VII, IX and X. Increased TTR leads to improved clinical outcomes and reductions in thromboembolic and hemorrhagic events. Patient self-testing and self-management through the use of a home INR monitor may be used to improve the time in therapeutic rate (TTR) for select groups of patients. Maintaining patients withinhis/her prescribed therapeutic range minimizes adverse events associated with inadequate or excessive anticoagulation such as serious bleeding or thromboembolic events. The INR is the ratio of the patient's PT (extrinsic or tissue-factor coagulation pathway) compared to the mean PT for a group of normal individuals. Use of the International Normalized Ratio (INR) or prothrombin time (PT) - standard measurement for reporting the blood's clotting time - allows physicians to determine the level of anticoagulation in a patient independent of the laboratory reagents used.


 0 kommentar(er)
0 kommentar(er)
